Welcome back, chemistry enthusiasts! Today, we’re diving into the fascinating world of acids, specifically focusing on two important ones: hydrochloric acid formula and fuming sulfuric acid. Join us as we explore their formulas, properties, and uses! Chemistry is all around us, from the air we breathe to the water we drink. One essential chemical compound that plays a crucial role in various industrial processes is hydrochloric acid. In this blog, we’ll delve into the basics of hydrochloric acid formula, and its applications in different industries.
Understanding Hydrochloric Acid:
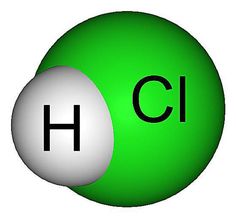
Hydrochloric acid, often referred to as HCl, is a powerful and versatile acid. Its formula is quite simple: HCl. Yes, it’s just made up of one hydrogen atom bonded to one chlorine atom. Despite its simplicity, don’t underestimate its potency!

Unveiling the Hydrochloric Acid Formula:
Let’s break down the formula further. The “H” stands for hydrogen, an essential element found abundantly in nature. The “Cl” represents chlorine, a highly reactive element known for its role in disinfectants and PVC production. When these two elements combine, they form a corrosive and acidic solution used in various industries.
Hydrochloric acid is composed of hydrogen and chlorine atoms. When hydrogen chloride gas dissolves in water, it forms hydrochloric acid. The chemical equation for this process is:
HCl (gas) + H2O (liquid) → H3O+ (aq) + Cl- (aq)
This equation illustrates how hydrogen chloride gas (HCl) reacts with water (H2O) to form hydronium ions (H3O+) and chloride ions (Cl-) in solution.
Exploring Hydrochloric Acid:
Hydrochloric acid is not just a laboratory chemical; it plays crucial roles in many aspects of our daily lives. From cleaning metal surfaces to regulating pH levels in swimming pools, its applications are vast and diverse. However, it’s essential to handle it with care due to its corrosive nature.
Introducing Fuming Sulfuric Acid:
Now, let’s shift our focus to another potent acid: fuming sulfuric acid. Also known as oleum, this compound is a mixture of sulfuric acid (H2SO4) and sulfur trioxide (SO3). Its formula may sound complex, but it’s essentially a combination of sulfur, oxygen, and hydrogen atoms.
The Power of Fuming Sulfuric Acid:
Fuming sulfuric acid is not your ordinary acid. Its ability to release toxic sulfur trioxide fumes makes it a valuable reagent in chemical synthesis. From the production of dyes and pharmaceuticals to petroleum refining, its applications are indispensable in various industries.
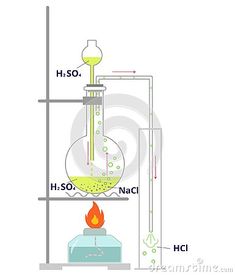
Applications of Hydrochloric Acid
Hydrochloric acid has numerous applications across different industries:
Chemical Industry: It is used in the production of organic and inorganic compounds, such as vinyl chloride for PVC production and chlorides for water treatment.
Metallurgy: Hydrochloric acid is employed in metal cleaning, pickling, and metal ore refining processes.
Food Industry: It is used for food processing, such as in the production of citric acid, food additives, and gelatin.
Textile Industry: Hydrochloric acid is utilized in textile dyeing and finishing processes.
Mining: It is employed in the extraction of minerals, such as the production of alumina from bauxite ore.
Safety Precautions
While hydrochloric acid is a valuable chemical in various industries, it is essential to handle it with care due to its corrosive nature. Safety precautions should always be observed when working with hydrochloric acid, including wearing appropriate protective equipment such as gloves, goggles, and lab coats. Additionally, hydrochloric acid should be stored and handled in well-ventilated areas to prevent the inhalation of its fumes.
Hydrochloric Acid Formula in Action: Case Study – Maruti Fine Chemicals
At Maruti Fine Chemicals, we specialize in the production and supply of high-quality hydrochloric acid for various industrial applications. Our state-of-the-art facilities ensure the precise formulation and purity of our hydrochloric acid, meeting the stringent requirements of our customers.
Conclusion:
Today, we’ve uncovered the basics of hydrochloric acid formula and delved into the intriguing world of fuming sulfuric acid. Remember, while these acids offer immense benefits, safety precautions should always be prioritized when handling them. Stay curious, and keep exploring the wonders of chemistry!
